Exciting News for Pediatric IBD Patients: Personalized Thiopurine Dosing Through Pharmacogenetics.
Inflammatory bowel disease (IBD) is a challenging condition affecting many children worldwide. The CDC estimates 1.3% of Adults are diagnosed with Ulcerative Colitis or Crohn's Disease. The incidence in pediatrics is even higher, ranging from 4% - 18%, depending on which age group you investigate. Thiopurine drugs, such as azathioprine and 6-mercaptopurine, have been widely used to manage IBD; however, they can cause severe side effects like myelosuppression (bone marrow suppression) in some patients. Recently released, there's great news you can use now!
Genetic Factors Affecting Thiopurine Response
The enzymes TPMT and NUDT15 play a crucial role in metabolizing thiopurines. Genetic variations in the genes encoding these enzymes can lead to reduced enzyme activity, increasing the risk of myelosuppression. Around 11% of pediatric IBD patients carry clinically actionable variants in either TPMT or NUDT15 that impact thiopurine dosing.
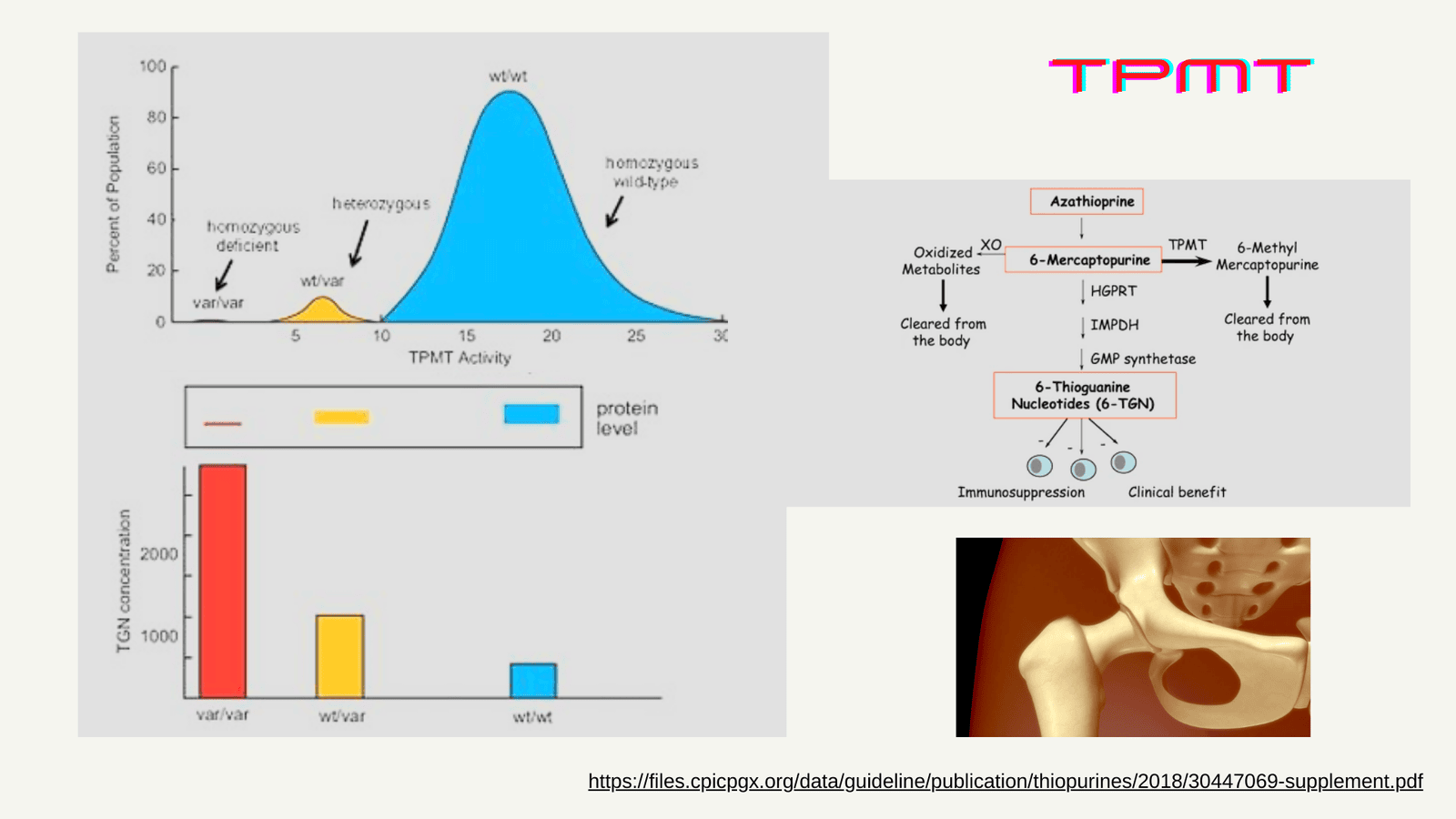
Updated CPIC Guidelines for Safer Thiopurine Use
The Clinical Pharmacogenetics Implementation Consortium (CPIC) has recently updated their guidelines, strongly recommending genotyping both TPMT and NUDT15 before initiating thiopurine therapy. Based on a patient's combined TPMT and NUDT15 genotypes, CPIC now provides personalized dosing recommendations:
- For poor metabolizers with non-functional TPMT or NUDT15 variants, alternative non-thiopurine therapy is advised due to the high risk of toxicity.
- Intermediate metabolizers require significant dose reductions (30-80%) from standard thiopurine doses.
This is a chart from the data available from this excel file on CPIC website, which displays the frequencies of the alleles in populations around the world.

https://zdrive.peregrineworx.com/sheet/publicgraphs/b3fa197870ec47c28746a28d5a223d551717041442754882
Preemptive Genotyping for Precision Medicine
This is a recent study in a diverse Canadian pediatric IBD cohort, which found patients with actionable NUDT15 variants were normal metabolizers for TPMT, and vice versa, highlighting the need for joint genotyping.
Preemptive joint TPMT/NUDT15 genotyping can identify high-risk patients and allow personalized, safer dosing or use of alternative therapies upfront. This proactive approach leverages pharmacogenomics to provide the best possible care for pediatric IBD patients before starting thiopurine treatment.
A Step Towards Personalized IBD Care
The updated CPIC guidelines emphasize the importance of comprehensive TPMT and NUDT15 genotyping prior to thiopurine treatment to optimize dosing across diverse ancestries and minimize the risk of potentially life-threatening myelosuppression.
As the global IBD burden rises, implementing these pharmacogenomic recommendations is a crucial step towards personalized, precision medicine for pediatric patients worldwide. Families and healthcare providers should be excited about this advancement, which promises to improve treatment safety and outcomes for children with IBD.
References:
1. https://stacks.cdc.gov/view/cdc/115240/cdc_115240_DS1.pdf
2. Kennedy AM, Griffiths AM, Muise AM, et al. Landscape of TPMT and NUDT15 Pharmacogenetic Variation in a Cohort of Canadian Pediatric Inflammatory Bowel Disease Patients. Inflamm Bowel Dis. Published online May 24, 2024:izae109. doi:10.1093/ibd/izae109
3. CPIC® Guideline for Thiopurines and TPMT and NUDT15 – CPIC. Accessed May 29, 2024. https://cpicpgx.org/guidelines/guideline-for-thiopurines-and-tpmt/
4. https://files.cpicpgx.org/data/report/current/frequency/TPMT_frequency_table.xlsx


